Soon here you will find the relevant information about the contradictions regarding the LXR-dependent induction of SREBP1.
The following article (and references therein) is an excellent example to obtain more detailed insight into this topic:
Duniec-Dmuchowski et al. Regulation of CYP3A4 and CYP2B6 expression by liver X receptor agonists. Biochem Pharmacol. 2007 Nov 15;74(10):1535-40. [»In transactivation assays, T0901317 activated human PXR and LXR with EC50s of 23 and 15 nM, respectively...« and »...T0901317 activated mouse PXR with an EC50 of 75 nM...«. Furthermore, for the relative quantification of SREBP1c mRNA levels, the authors used a TaqMan Gene Expression Assay, Hs00231674_m1, that is SREBP1a isoform-specific. For this reason, there are two options: T0901317 activates PXR in the low nanomolar range which leads to the induction of SREBP1a or LXR induces the transcription of SREBP1a]
However, it is much more important
to determine the main factor which is
responsible for the elevated (preprandial) de novo lipogenesis in NAFLD/NASH -
And in this context, it seems necessary to
leave the conventional view (SREBP1c) and develop New Conceptions (SREBP1a).
The following animation scheme and the associated articles not only support this statement, but also clearly demonstrate that it is highly probable that...
...SREBP1a Is The Main Factor.
Details will follow - please scroll down...
Alternative Concept for the Regulation of Cholesterol and Fatty Acid Metabolism in Human Liver
Time for a paradigm shift?
In 1994, Nobel Laureates Goldstein & Brown and co-authors have published the following article:
They have concluded (1994!): »Sterols inhibit the cleavage of SREBP-1, and the 68 kd nuclear form is rapidly catabolized, thereby reducing transcription.«
And this dogma is still applied today:
»Sterols inhibit the cleavage of the precursor, and the mature nuclear form is rapidly catabolized, thereby reducing transcription.« (see ref 1, ref 2, ref 3)
However, the results of dozens of scientific articles contradict and/or do not support this dogma. The literature analysis on srebp1a.com and the results of the following mentioned article reveal it.
Before reading further, please note that:
- 1. HMGCR is the rate-limiting enzyme of sterol biosynthesis.
- 2. HMGCR activity (protein) do not repress, but induces the maturation/cleavage of SREBP1. (see ref 4, ref 5, ref 6)
- 3. In the early 1990s, Nobel Laureates Goldstein & Brown discovered SREBPs (SREBP1a, SREBP1c and SREBP2) as key transcriptional regulators of lipid metabolism. However, one and a half years ago, the only "synonyms" for the SREBF1 gene were SREBP1, bHLHd1 and SREBP-1c (provided by NCBI and HGNC). SREBP1a could not be found! This “mistake” was corrected in February 2016, after I have mentioned it. See here: NCBI and HGNC.
The Strength of the Connection between Cholesterol and Fatty Acid Metabolism (Part 1 of 3)
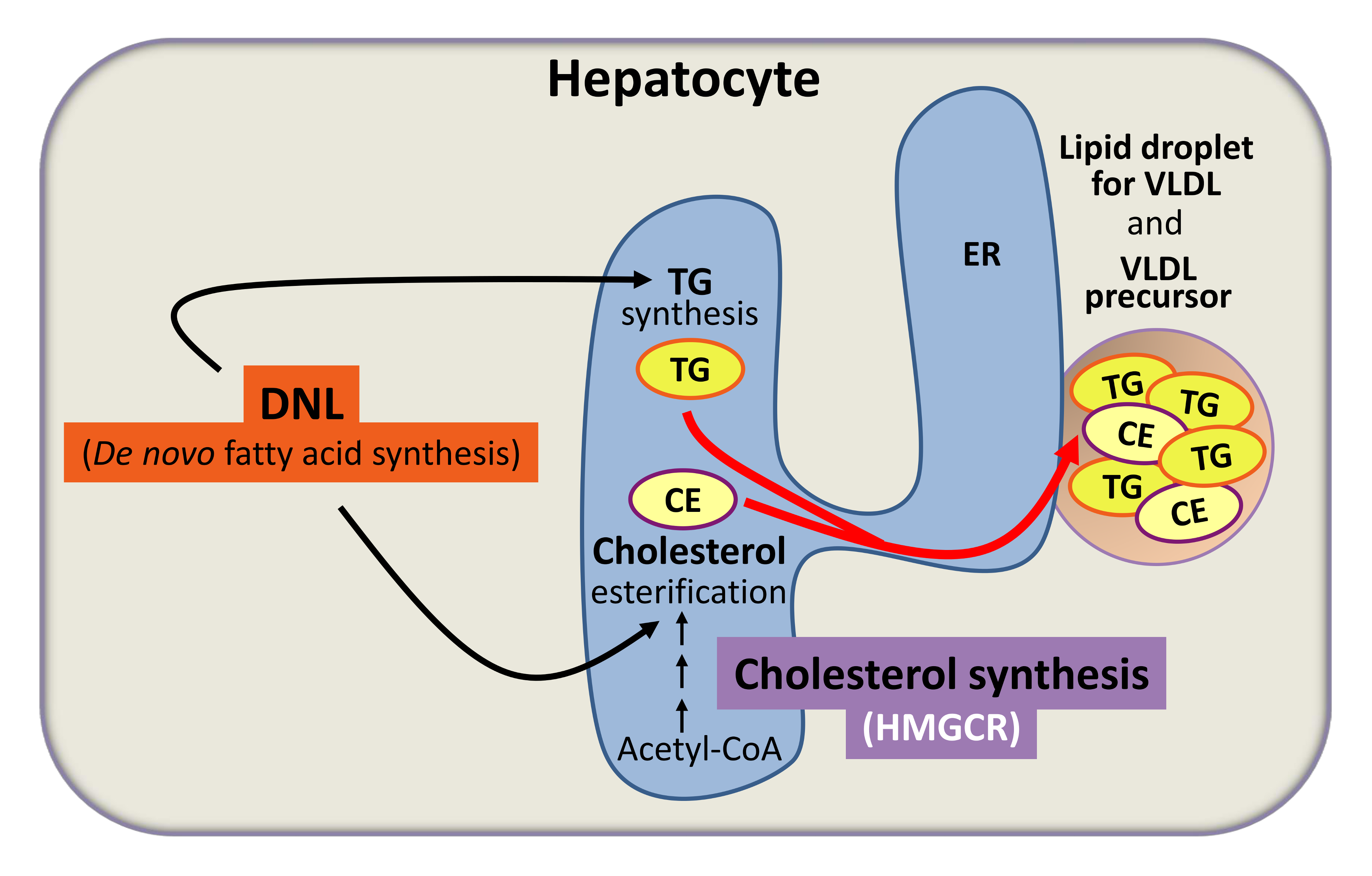 1. De novo synthesized fatty acids for VLDL-anabolism (simplified scheme). DNL, de novo lipogenesis; TG, triglyceride; CE, cholesteryl ester; ER, endoplasmic reticulum; VLDL, very low density lipoprotein; HMGCR, HMG-CoA reductase.
1. De novo synthesized fatty acids for VLDL-anabolism (simplified scheme). DNL, de novo lipogenesis; TG, triglyceride; CE, cholesteryl ester; ER, endoplasmic reticulum; VLDL, very low density lipoprotein; HMGCR, HMG-CoA reductase.
Very-low-density lipoprotein (VLDL), made by the liver in hepatocytes, delivers fatty acids and cholesterol in form of triglycerides and cholesteryl esters to cells throughout the body. De novo synthesized fatty acids in hepatocytes are important determinants for hepatic triglyceride synthesis and cholesterol esterification (see Fig. 1 above).
The question is:
Do or how do fatty acid and cholesterol metabolism interact witch each other in hepatocytes? - A groundbreaking new regulatory circuit?
HMGCR and SREBP1a: Interplay of Cholesterol and Fatty Acid Metabolism (Part 2 of 3)
2. HMGCR-dependent SREBP1a maturation (simplified animation scheme). 1a, mature SREBP1a protein; PUFA, polyunsaturated fatty acid; ER, endoplasmic reticulum.