The Pathogenesis of NASH: What about PXR, SREBP1a and AKR1B10?
A Future Research Project
(created: 2015)
1. Question and state of research
Today's lifestyle in the developed world, characterized by overeating and lack of exercise, leads to impaired lipid and glucose metabolism. As a result, obesity and type 2 diabetes mellitus (T2D) have reached epidemic proportions and are a global health problem. It is estimated that 500 million adults worldwide are obese and around 300 million people suffer from T2D [1, 2]. Hyperinsulinemia, hyperglycemia and hypertriglyceridemia, metabolic disorders which are strongly associated with obesity and T2D, are often associated with chronic liver diseases [3, 4, 5, 6]. Highly important is nonalcoholic fatty liver disease (NAFLD), with an alarmingly high global prevalence of 20-30% [7, 8, 9]. The term NAFLD covers a wide range of liver diseases, with the simple fatty degeneration of liver cells (steatosis) representing a reversible disease state which, however, develops into nonalcoholic steatohepatitis (NASH) in 30-40% of all cases [10, 11]. In addition to steatosis, fibrosis and a ballooning of hepatocytes, the chronic inflammation of the liver is a characteristic feature of NASH [12, 13]. In approximately 20% of NASH patients the progressive form of NAFLD leads to the development of cirrhosis which may progress to hepatocellular carcinoma (liver cancer) [10, 14]. The epidemic nature of obesity and T2D, and the fact that up to 80% of overweight people and about 80% of T2D patients have NASH [15, 16], suggests that NAFLD may be the leading cause of severe liver disease in the years to come and NASH cirrhosis is likely to be the main indication for liver transplantation [7, 17, 18]. The severity of steatosis has been shown to be positively associated with NASH [19]. In addition, NAFLD is a strong predictor of prediabetes (insulin resistance) [20], the precursor of T2D, with the risk of prediabetes increasing with the severity of steatosis [21, 22, 23]. Therefore, hepatic insulin resistance and T2D are already considered to be secondary to NAFLD [24] and there is a great deal of interest in understanding the complex processes involved in the development of steatosis and, consequently, designing new treatment approaches [18].
The common histological feature of simple fatty liver and NASH is the excessive storage of triglycerides, in the form of lipid droplets, in hepatocytes (steatosis). So far, the increased release of fatty acids (lipolysis) from adipose tissue in NAFLD patients has been considered to be a major factor in the development of hepatic steatosis [25]. However, recent studies have shown that the pathophysiologically increased hepatic fatty acid synthesis (hepatic de novo lipogenesis) is the main characteristic of NAFLD [26]. Normally, the newly synthesized fatty acids in the liver account for only about 5% of hepatic triglyceride synthesis. This proportion increases to 23% after a meal [27]. In NAFLD patients, on the other hand, this proportion is already 26% in the fasted state and there is no further postprandial increase [28]. This suggests that NAFLD patients, independent of food intake and therefore insulin-independent, have a permanent and maximally increased hepatic de novo lipogenesis (DNL). Interestingly, increased DNL is also associated with the co-morbidities typically associated with NAFLD, such as obesity, insulin resistance and T2D [29, 30, 31, 32]
To better understand the complex interdependence between the civilization diseases NAFLD, obesity and T2D, the factors must be identified which contribute to the development and maintenance of increased hepatic DNL and also identify the pathophysiologically relevant effects of a chronically elevated hepatic DNL that may have all of these diseases in common.
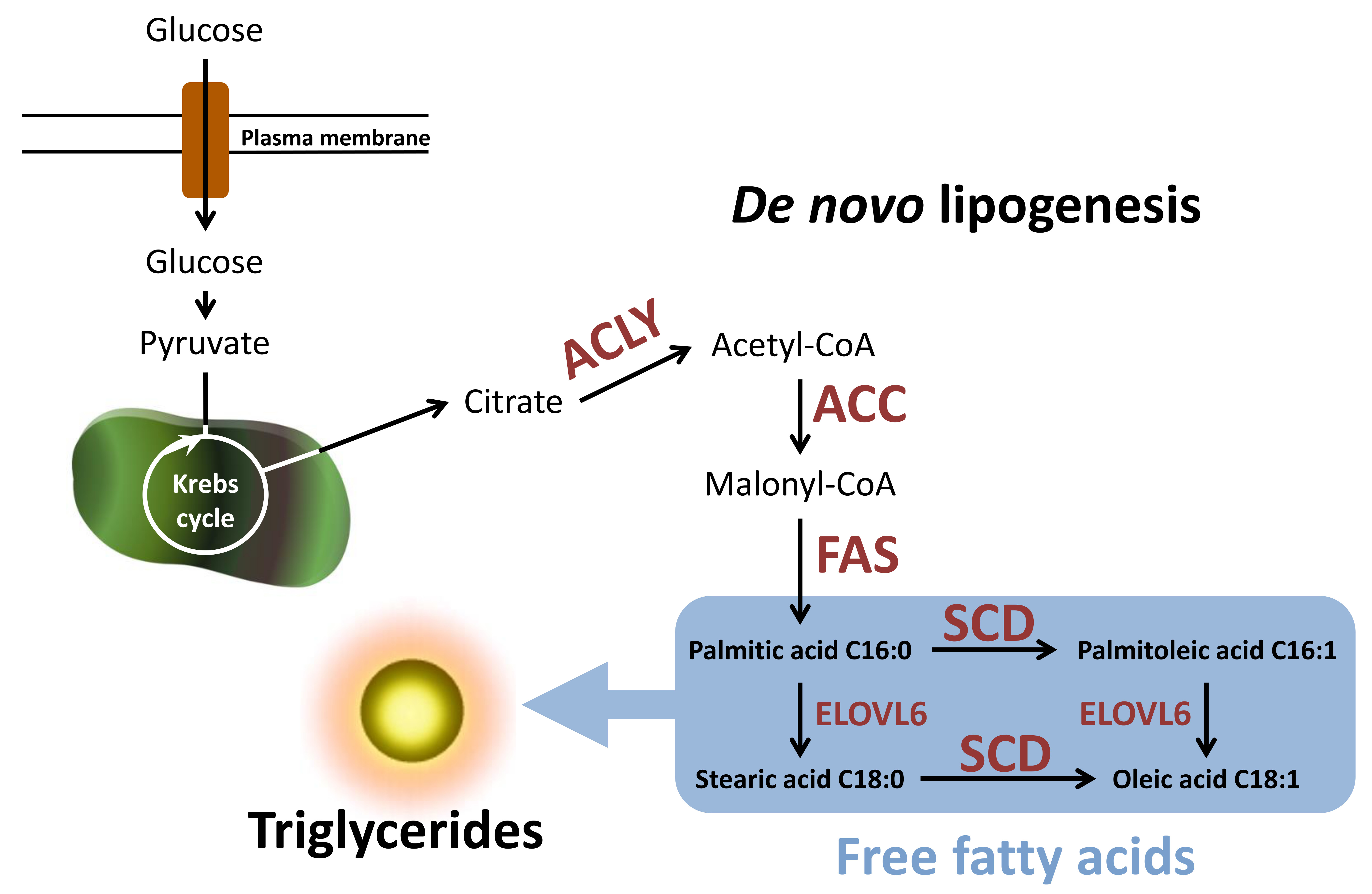 Figure 1: Simplified scheme of de novo lipogenesis (DNL) and its most important reaction steps. Glucose is used for the new synthesis of fatty acids and finally accumulates in the form of triglycerides in the cell. (for more details, please scroll down)
Figure 1: Simplified scheme of de novo lipogenesis (DNL) and its most important reaction steps. Glucose is used for the new synthesis of fatty acids and finally accumulates in the form of triglycerides in the cell. (for more details, please scroll down)
The glucose and lipid metabolism are closely related (Figure 1). At high substrate availability (e.g., glucose), an intermediate from the citric acid cycle, citrate, is used for the new synthesis of fatty acids and ultimately accumulates in the form of triglycerides in the cell. Acetyl-CoA, the parent molecule of DNL, is formed by enzymatic cleavage of citrate using ATP citrate lyase (ACLY). The following reaction steps of DNL are catalyzed by the key lipogenic enzymes acetyl-CoA-carboxylase 1 (ACC1) and fatty acid synthase (FAS). ACC1 catalyzes the first rate-limiting reaction in DNL, with malonyl-CoA formed by the carboxylation of acetyl-CoA and the FAS serves as a substrate for the new synthesis of fatty acids. The resulting palmitic acid can be metabolized to higher-chain or monounsaturated fatty acids by two other key enzymes of fatty acid synthesis, fatty acid elongase 6 (ELOVL6) and stearoyl-CoA-desaturase (SCD).
The transcriptional regulation of gene expression of DNL-essential enzymes (ACLY, ACC1, FAS, ELOVL6, and SCD) is strongly influenced by the sterol regulatory element-binding protein 1 (SREBP1) [33, 34]. As a consequence of increased expression of this transcription factor, which is most important for fatty acid synthesis, increased levels of triglycerides are stored in the liver [35]. Correspondingly, hepatic expression of SREBP1 was increased in NAFLD patients in several clinical studies [36, 37, 38, 39, 40, 41]. Another possible relevant factor could be the aldo-keto-reductase (AKR) 1B10-dependent accumulation of ACC1. In extrahepatic cell lines, it has been shown that AKR1B10 and ACC1 undergo a protein-protein interaction, thereby protecting ACC1 from proteasomal degradation [42]. Thus, siRNA-mediated knockdown of human AKR1B10 leads to a decrease in the protein content of ACC1 and a concomitant reduction of the new synthesis of lipids [42]. NASH patients show increased hepatic expression of AKR1B10 compared to healthy individuals [41, 43]
In summary, it can be concluded that the factor that has so far been negligible with regard to the development and worsening of NAFLD and T2D, the increased hepatic de novo lipogenesis, must now be regarded as one of the main factors in the pathogenesis of NAFLD and T2D. Consequently, in view of the ever-increasing incidence of NAFLD and T2D, the main focus should be on the functional analysis of the signaling pathways or genes that contribute to the development, maintenance and pathophysiological consequences of increased hepatic DNL.
2. Own preparatory work
2.1. The dual role of PXR in human hepatic de novo lipogenesis
Own publication:
Bitter et al. Pregnane X receptor activation and silencing promote steatosis of human hepatic cells by distinct lipogenic mechanisms. Arch Toxicol. 2015 Nov; 89 (11): 2089-103. (See Figure 1-7 via SemanticScholar)
The special property of the nuclear receptor pregnane X receptor (PXR, NR1I2) to be activated by the most diverse xenobiotics, is consistent with its physiological role to protect the body from potentially dangerous and toxic substances. By ligand-dependent activation, PXR induces the metabolism and transport of foreign substances and thus counteracts their harmful accumulation. In addition to the classical target genes of PXR, such as CYP3A4 and MDR1, the expression of a variety of other genes is influenced by the activation or deficiency of PXR. Results based on in vivo studies increasingly indicate that modulation of the expression or activity of the nuclear receptor PXR represents a potential therapeutic treatment option for NAFLD and/or T2D patients. PXR affects both glucose and lipid homeostasis by inhibiting or activating the expression of key genes in gluconeogenesis, the uptake and β-oxidation of fatty acids, and de novo lipogenesis [44, 45, 46, 47, 48, 49]. In mice, both ligand-dependent activation and knockout of PXR lead to hepatic steatosis [44, 45, 50]. To what extent these findings can be transferred to humans was largely unexplained. However, in view of the in vivo studies already mentioned and the fact that single nucleotide polymorphisms (SNPs) in the PXR gene are associated with the severity of NAFLD [51], the nuclear receptor PXR could play an important role in the pathogenesis and progression of the disease NAFLD, and consequently in human hepatic steatosis.
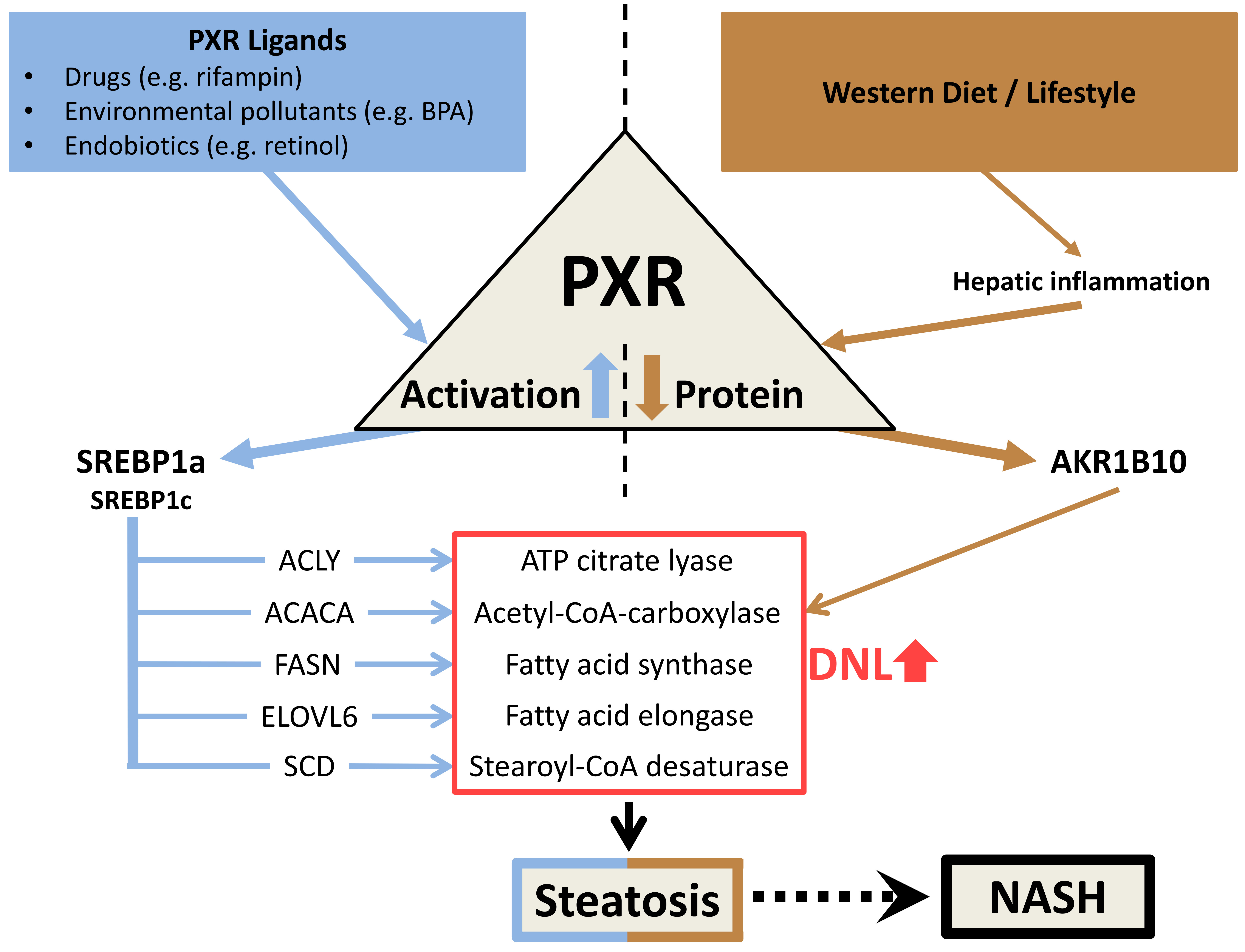 Figure 2: Scheme of the potential dual role of PXR in the development of human hepatic steatosis. (for more details, please scroll down)
Figure 2: Scheme of the potential dual role of PXR in the development of human hepatic steatosis. (for more details, please scroll down)
I could show for the first time that in human hepatic cells (HepG2 and primary human hepatocytes) a ligand-mediated activation of PXR, via the induction of SREBP1a, leads to the initiation of the SREBP1-dependent lipogenic signaling pathway and a deficiency of PXR increases the efficiency of the ACC-catalyzed reaction step of de novo lipogenesis by means of increased expression of AKR1B10. Both PXR-dependent molecular mechanisms stimulate the de novo synthesis of fatty acids (DNL, de novo lipogenesis), thereby promoting the development and enhancement of human hepatic steatosis (Figure 2) [41].
Furthermore, for the first time, I have shown that NASH livers contain about 60% less PXR protein compared to non-NASH livers [41]. Correspondingly, I was able to confirm the results of Starmann et al. [43], an elevated hepatic expression of AKR1B10 in NASH patients, by analyzing another cohort [41]. My established cell culture model, based on stable clones of the HepG2 hepatic cell line, showing high and low PXR expression [41], is comparable to the difference in PXR protein content in NASH livers and non-NASH livers. Despite the PXR knockdown cell's approximately 60% lower PXR protein content, expression of SREBP1a induced by ligand-mediated PXR activation was also observed in these HepG2 clones. This suggests that the increased hepatic expression of SREBP1a observed in NASH patients (see 2.2) may result from chronic activation of the nuclear receptor PXR, despite the lower level of PXR protein.
The cell culture model I have established may represent the PXR-dependent pathophysio-logically relevant situation of permanent and maximally increased hepatic de novo lipogenesis in NASH patients. In this "in vitro NAFLD model", the condition of PXR deficiency which occurs at the same time with increased ligand-mediated PXR activity (Figure 3, state 3), leads to maximally enhanced lipid droplet formation (Figure 3 - left panel) and maximally increased accumulation of triglycerides (Figure 3 - right panel).
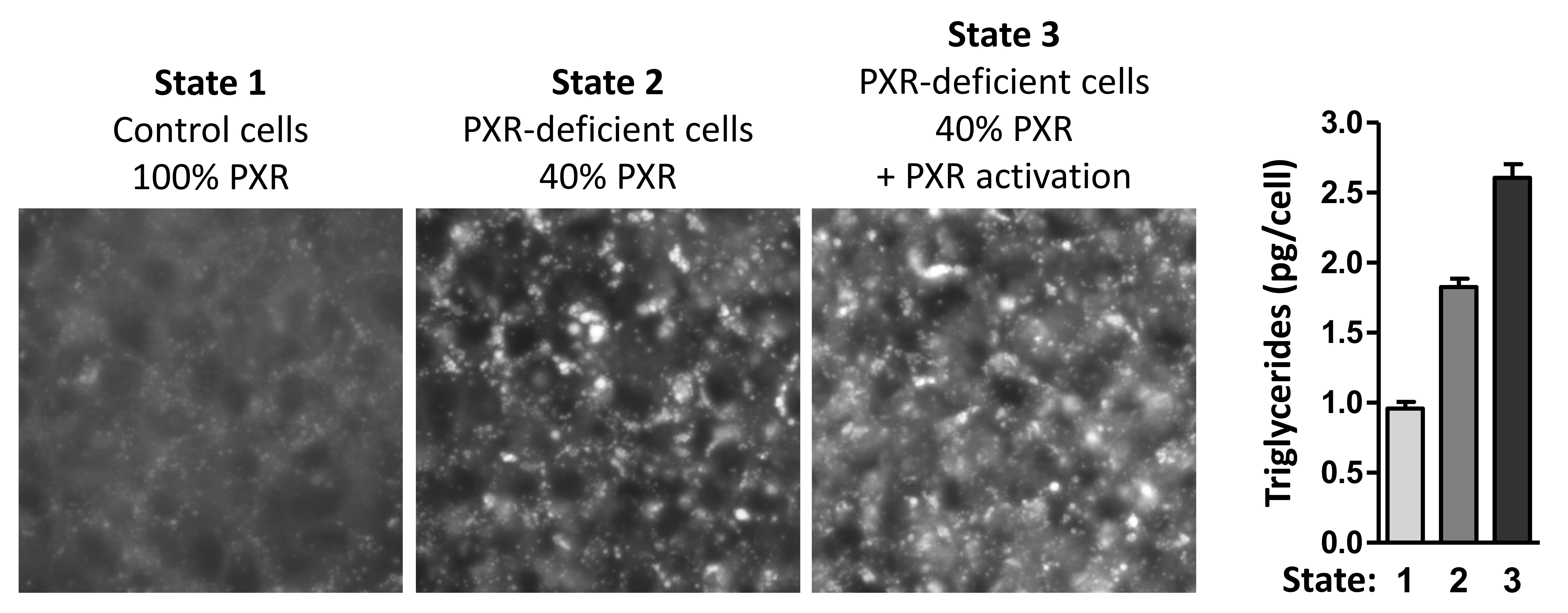 Figure 3: The combination of ligand-mediated PXR activation and PXR deficiency leads to the highest achievable hepatic steatosis. (for more details, see above)
Figure 3: The combination of ligand-mediated PXR activation and PXR deficiency leads to the highest achievable hepatic steatosis. (for more details, see above)
2.2 The role of SREBP1a in human hepatic de novo lipogenesis
Own publication:
Bitter et al. Human sterol regulatory element-binding protein 1a contributes significantly to hepatic lipogenic gene expression. Cell Physiol Biochem. 2015;35(2):803-15.
The transcription factor SREBP1 exists in two isoforms, 1a and 1c. Because of the longer transactivation domain, SREBP1a has approximately 10-fold greater transcriptional activity than SREBP1c [52, 53]. However, based on studies in the mouse, it has been suggested that SREBP1a is also much less expressed in the human liver than SREBP1c [54]. Therefore, SREBP1a was considered to be negligible in transcriptional regulation of human hepatic DNL and has not been explicitly considered in any study to investigate the role of SREBP1 in human DNL or NAFLD. In my recent work on the role of SREBP1a in human hepatic DNL and NASH, it has been demonstrated for the first time that livers of NASH patients show markedly increased expression of the isoform SREBP1a and its major lipogenic target genes ACLY, ACACA, FASN, ELOVL6 , SCD and THRSP [40,41]. Furthermore, the specific knockdown of SREBP1a in primary human hepatocytes (PHHs) negatively affected the gene expression of the DNL essential enzymes ACC (-25%), FAS (-40%) and SCD (-35%) and also resulted in about 50% lower levels of SREBP1c whose gene expression is autoregulated by SREBP1. Surprisingly, gene expression of these lipogenic enzymes was not more strongly affected by siRNA-mediated knockdown of total SREBP1 (1a + 1c). These results strongly suggest that the isoform SREBP1a has an important regulatory role in human hepatic DNL. Furthermore, for the first time in a large cohort, I analyzed the relative ratio of SREBP1c to SREBP1a in human livers and PHHs. I found that the relative ratio of SREBP1c to SREBP1a, both in the human liver (approximately 3 to 1) and in PHHs (approximately 2 to 1), by no means matches the already known relative mouse ratios (approximately 10 to 1) [40]. In summary, it can be concluded that the human isoform SREBP1a, in clear contrast to the murine, contributes considerably more to the total hepatic SREBP1 content and has a greater influence on the gene expression of hepatic DNL essential enzymes. Accordingly, and given the increased expression of SREBP1a and its major lipogenic target genes in NASH livers, the question arises how the expression of SREBP1a in the human liver is physiologically regulated and, more importantly, pathophysiologically influenced by which relevant factors.
3. Hypotheses
The higher expression of SREBP1a in NASH livers and the resulting higher expression of lipogenic SREBP1 target genes suggests that the nuclear receptor PXR could be chronically activated in NASH patients. Characteristic of liver fibrosis, a major feature of the pathogenesis and progression of NASH [12, 13], is liver damage-induced activation of hepatic stellate cells (HSCs) [55, 56] which are the main storage site for retinol in the body [57]. As a result of activation of hepatic stellate cells, this retinyl ester (RE) stored retinol, a potent endogenous PXR ligand [58, 59], is released [60]. Consequently, it can be assumed that even a mild NASH with concomitant mild fibrosis leads to chronic retinol-mediated activation of the nuclear receptor PXR and, subsequently, the expression of SREBP1a and lipogenic SREBP1 target genes in hepatocytes is pathophysiologically elevated. The following results support this hypothesis: 1) Livers with mild NASH already have increased gene expression of SREBP1a, ACC1, FAS and SCD compared to non-NASH livers. The steatosis grade is correspondingly increased by 2.5 times [41]. 2) Physiologically relevant concentrations of [59] induce concentration-dependently the expression of SREBP1a in PXR-overexpressing HepG2 cells (clone H-P). In addition, I found that, in synchronism with the induction of SREBP1a, the expression of the PXR target gene CYP24A1, characteristic for ligand-induced activation of PXR in HepG2 cells, was increased in a concentration-dependent manner by in H-P cells. 3) Mice which received a high fat diet develop the typical features of NAFLD/NASH, and liver damage or the activation of hepatic stellate cells increases in this murine NAFLD/NASH model with time steadily [60, 61, 62, 63, 64, 65]. Interestingly, the hepatic effects of this high-fat diet (hepatic steatosis, increased liver triglyceride content, hepatic inflammation, increased hepatic expression of SREBP1 and its lipogenic target genes Acc, Fas and Scd) are markedly lower in PXR knockout mice compared to wild-type mice [66].
Accordingly, and assuming that retinol released from activated hepatic stellate cells (HSCs) due to liver injury increases the activity of the nuclear receptor PXR in hepatocytes, it can be concluded that the expression of SREBP1a, the expression of lipogenic target genes of SREBP1a, the activity of hepatic DNL and, ultimately, the severity of steatosis in NASH livers is PXR-dependent.
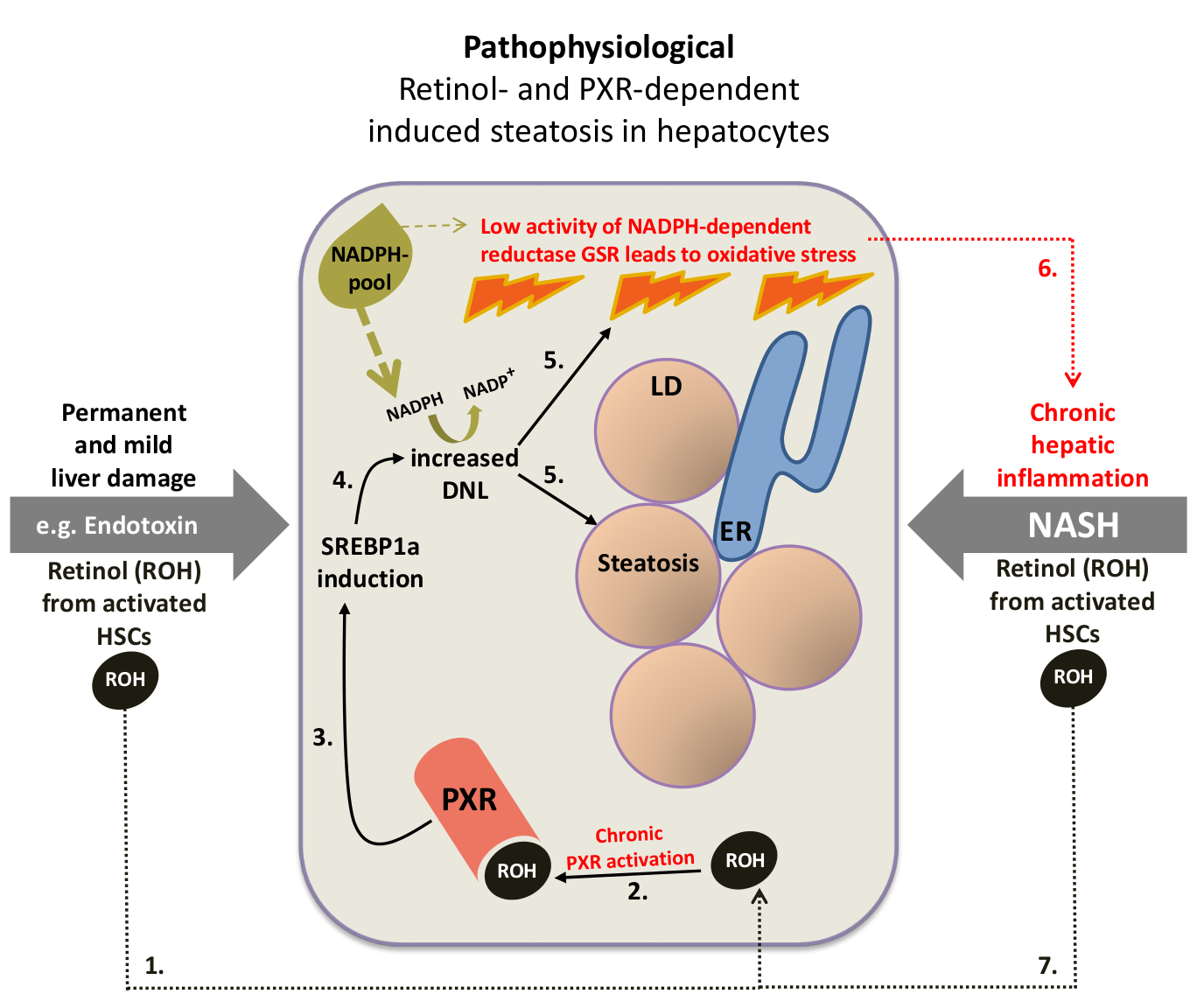 Figure 4: Hypothetical scheme of the pathophysiological role of retinol-mediated activation of the pregnane X receptor in the development and progression of nonalcoholic steatohepatitis. DNL, de novo lipogenesis; LD, lipid droplet; ER, endoplasmic reticulum; HSC, hepatic stellate cell; GSR, glutathione reductase. Numbers indicate the proposed sequence of events. (for more details, please scroll down)
Figure 4: Hypothetical scheme of the pathophysiological role of retinol-mediated activation of the pregnane X receptor in the development and progression of nonalcoholic steatohepatitis. DNL, de novo lipogenesis; LD, lipid droplet; ER, endoplasmic reticulum; HSC, hepatic stellate cell; GSR, glutathione reductase. Numbers indicate the proposed sequence of events. (for more details, please scroll down)
Hepatic oxidative stress, the result of a pathophysiologically high concentration of reactive oxygen species (ROS), is considered to be a key component in the pathogenesis of NASH and hepatic insulin resistance and may therefore explain the strong association between NASH and T2D. The capacity of hepatocytes to eliminate reactive oxygen species, e.g. the superoxide anion radical •O2- and hydrogen peroxide (H2O2), and thus to counteract cell or tissue damage, is determined by the activity of a variety of antioxidant enzymes. In contrast to •O2- which is rapidly converted to H2O2, the more stable H2O2 has the ability to diffuse through cell membranes, thereby damaging adjacent cells as well. The elimination of an amount of H2O2 exceeding the physiological extent is mainly determined by the activity of the NADPH-dependent enzyme glutathione reductase (GSR).
For the de novo synthesis of one molecule of palmitic acid, the primary product of the new synthesis of fatty acids (see Figure 1), the cell requires 14 molecules of NADPH. Therefore, I assume that as soon as the permanently increased DNL reaches a certain threshold, at which the cellular capacity to replenish the NADPH pool is exceeded, the activity of the NADPH-dependent enzyme GSR, essential for the elimination of H2O2, is decisively reduced which eventually leads to oxidative stress (Figure 4, sequence of events 1 to 5). The resulting oxidative stress could trigger a positive feedback (circulus vitiosus) which increases the severity of liver damage and eventually leads to the disease state of NASH, a chronic hepatic inflammation with concomitant hepatic steatosis (Figure 4, recurring sequence of events 6->7->2->3->4->5->6).
The ligand-mediated activation of PXR-induced metabolism and transport of toxic substances requires both, NADPH for the activity of the xenobiotic metabolising cytochrome P450 enzymes and ATP for the activity of the efflux transporter. Therefore, it is only natural that, in parallel, activation of PXR leads to the inhibition of other energy-consuming metabolic processes, such as gluconeogenesis. It is all the more astonishing that the DNL, which requires 14 NADPH molecules for the new synthesis of only one palmitic acid molecule, is also stimulated by the ligand-mediated activation of PXR via the induction of SREBP1a. This may indicate an indispensable physiologically relevant role of PXR-dependent induced steatosis. Interestingly, PXR knockout mice and genetically engineered mice lacking the ability to store retinol in hepatic stellate cells (lrat-knockout mice) have common features after partial hepatectomy [67,68]. Compared to the corresponding wild-type mice, both genetically modified mice have a lower liver triglyceride content dependent on the liver resection and a low proliferation rate of the hepatic cells. Energy stored in the form of triglycerides (lipid droplets / "physiological" steatosis) is believed to be essential for optimal liver regeneration [67, 69]. Correspondingly, in both knockout mouse models, liver regeneration is compromised. Compared to PXR knockout mice, hepatic resection-dependent expression of both the mouse prototypic PXR target gene Cyp3a11 and the expression of the DNL and SREBP1a-regulated genes Acaca and Elovl6 were conspicuously increased in wild-type mice. In addition, it was only in wild-type mice that a pronounced steatosis triggered by partial hepatectomy was observed. These results suggest that an endogenous PXR ligand induced by acute hepatic injury, possibly the retinol (ROH) stored in the hepatic stellate cells in the form of retinyl ester (RE), increases the activity of PXR to generate an important "physiological" steatosis for liver regeneration (Figure 5B). In the healthy liver, the retinol (ROH) stored in the form of retinyl ester (RE) remains in hepatic stellate cells and only a small amount of retinol is transferred to hepatocytes (Figure 5A). The retinol that has entered the hepatocytes is metabolised to retinal (RAL) and further to retinoic acid. This metabolic pathway is not affected during acute liver regeneration (Figure 5B) [68]. However, the increased hepatic expression of AKR1B10 in NASH patients, possibly due to the low protein content of PXR, could significantly alter the retinol metabolism in hepatocytes. Because of the high catalytic activity of AKR1B10 to convert retinal to retinol [70, 71], hepatocytes from NASH patients may show a pathophysiologically relevant accumulation of retinol, maximally and permanently increasing PXR activity (Figure 5C). The resulting potential consequences for the development and progression of NASH have already been explained using Figure 4. Therefore, PXR deficiency, by means of increased expression of AKR1B10, presumably not only leads to increased efficiency of the ACC-catalyzed reaction step of de novo lipogenesis, but also, due to the increased accumulation of retinol, to ligand-dependent stimulation of PXR activity.
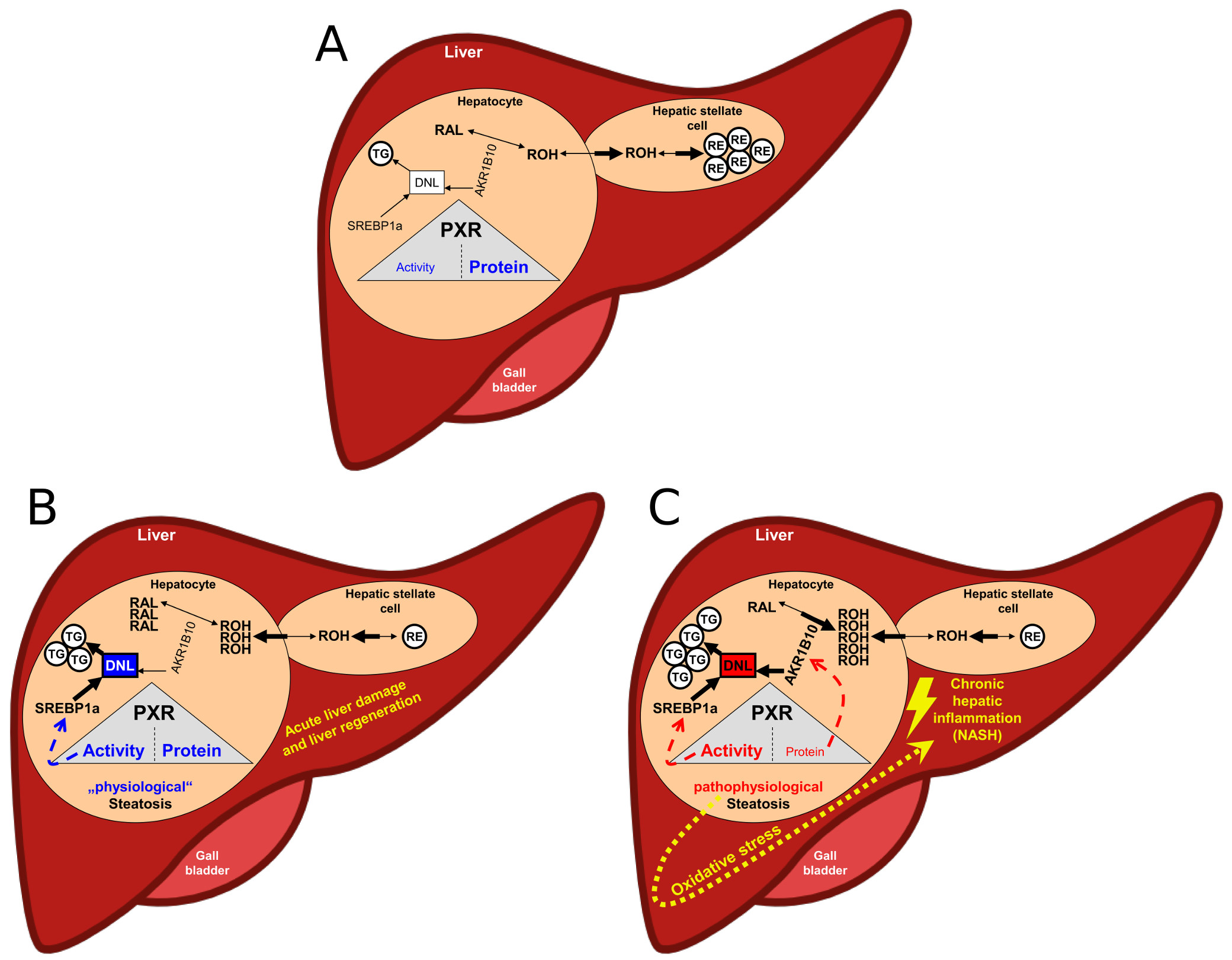 Figure 5: Hypothetical scheme of the physiological (B) and pathophysiological (C) PXR-dependent hepatic steatosis. A) Normal condition of the liver: low ligand-mediated PXR activity and high/stable protein content of PXR. RAL, retinal; ROH, retinol; RE, retinyl ester; TG, triglyceride; DNL, de novo lipogenesis. (for more details, see above)
Figure 5: Hypothetical scheme of the physiological (B) and pathophysiological (C) PXR-dependent hepatic steatosis. A) Normal condition of the liver: low ligand-mediated PXR activity and high/stable protein content of PXR. RAL, retinal; ROH, retinol; RE, retinyl ester; TG, triglyceride; DNL, de novo lipogenesis. (for more details, see above)