
Interspecies Differences in SREBP1 Signaling
Posted 7/28/2018
»Whereas decreased phosphorylation of AMPK and modulation of a series of downstream events leading to fatty acid synthesis and lipogenesis were observed in rat hepatocytes and human hepatoma cell lines, mouse hepatocytes were resistant to OA with regard to the AMPK/SREBP-1-dependent lipogenic pathway. Similar results were observed in animal studies using SD rats and C57BL/6 mice. These two species showed completely different responses to OA in terms of AMPK/SREBP-1 signaling and development of steatosis, indicating that not only pharmacokinetic but also pharmacodynamic factors participate in determining interspecies differences.«
Mouse models of human disease: An evolutionary perspective.
Posted 4/18/2018
Perlman RL. Mouse models of human disease: An evolutionary perspective. Evol Med Public Health. 2016 May 21;2016(1):170-6.
Abstract
The use of mice as model organisms to study human biology is predicated on the genetic and physiological similarities between the species. Nonetheless, mice and humans have evolved in and become adapted to different environments and so, despite their phylogenetic relatedness, they have become very different organisms. Mice often respond to experimental interventions in ways that differ strikingly from humans. Mice are invaluable for studying biological processes that have been conserved during the evolution of the rodent and primate lineages and for investigating the developmental mechanisms by which the conserved mammalian genome gives rise to a variety of different species. Mice are less reliable as models of human disease, however, because the networks linking genes to disease are likely to differ between the two species. The use of mice in biomedical research needs to take account of the evolved differences as well as the similarities between mice and humans.
The Blinded Scientific Community - Built up a Murine Hospital???
Posted 4/17/2018
What is going on?
Q?
Future proves Past
Be strong (anons)
Ready for paradigm shift?
Posted 8/22/2017
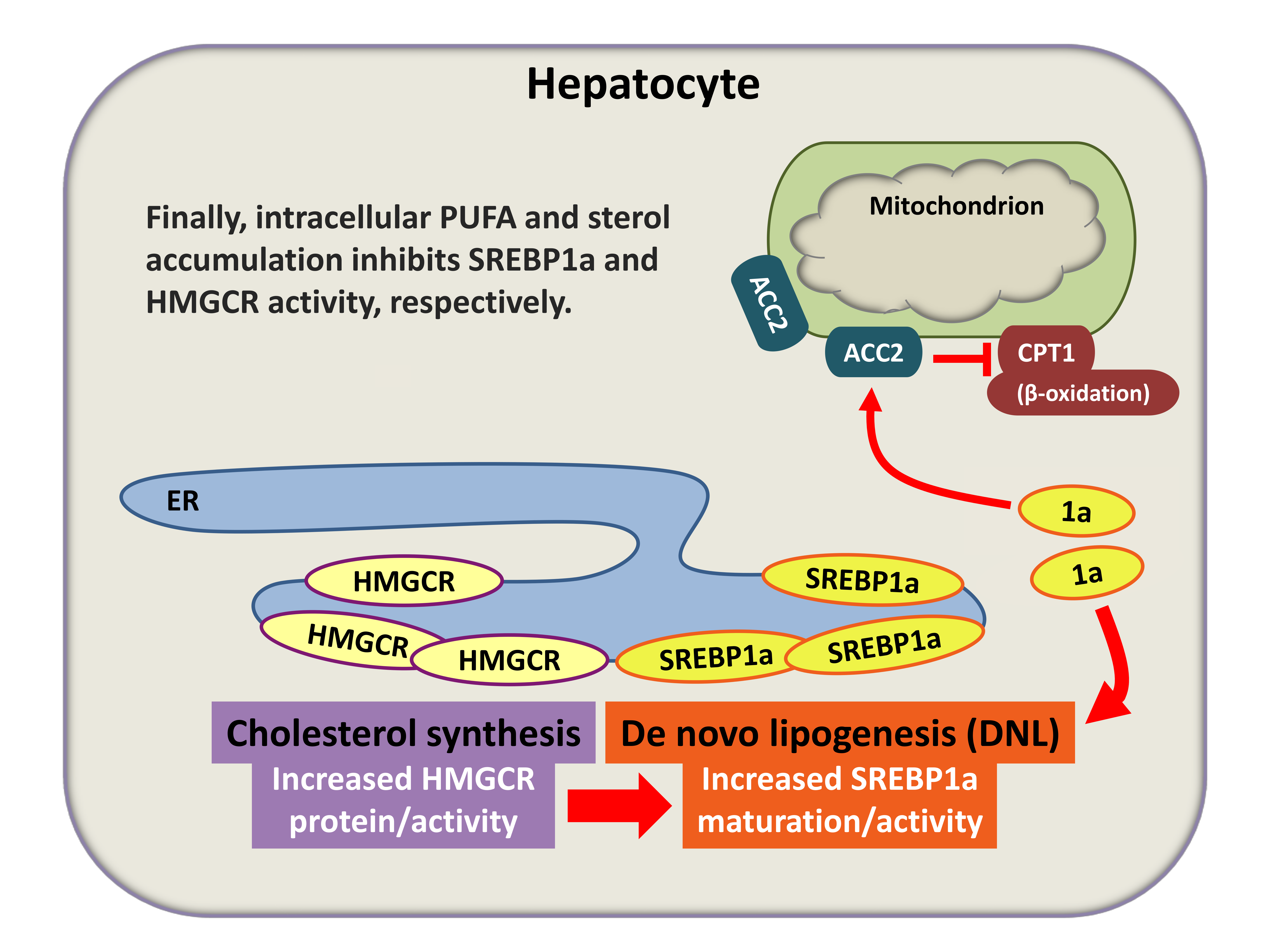 HMGCR-dependent SREBP1a maturation (simplified scheme). 1a, mature SREBP1a protein; ER, endoplasmic reticulum. (see section »PXR versus LXR«)
HMGCR-dependent SREBP1a maturation (simplified scheme). 1a, mature SREBP1a protein; ER, endoplasmic reticulum. (see section »PXR versus LXR«)
The dogma of the SREBP1 protein:
»Sterols inhibit the cleavage of the precursor, and the mature nuclear form is rapidly catabolized, thereby reducing transcription.« (see ref 1, ref 2, ref 3)
However, with all due respect, I would like to mention that the results of dozens of scientific articles contradict and/or do not support this dogma. The literature analysis on srebp1a.com and the results of the following mentioned article reveal it.
Before the literature analysis on srebp1a.com and the analysis of the article mentioned above, please consider the following two points:
1. HMGCR is the rate-limiting enzyme of sterol biosynthesis.
2. HMGCR protein/activity induces the maturation of SREBP1.
Time is running out fast
Posted 8/13/2017
Alarming predictions:
Abstract
BACKGROUND:
Nonalcoholic fatty liver disease (NAFLD) and resulting nonalcoholic steatohepatitis (NASH) are highly prevalent in the US, where they are a growing cause of cirrhosis and hepatocellular carcinoma (HCC), and increasingly, an indicator for liver transplantation.
METHODS:
A Markov model was used to forecast NAFLD disease progression. Incidence of NAFLD was based on historical and projected changes in adult prevalence of obesity and type 2 diabetes mellitus (DM). Assumptions were derived from published literature where available, and validated using national surveillance data for incidence of NAFLD-related HCC. Projected changes in NAFLD-related cirrhosis, advanced liver disease, and liver-related mortality were quantified through 2030.
RESULTS:
Prevalent NAFLD cases are forecasted to increase 21%, from 83.1 (2015) to 100.9 million (2030), while prevalent NASH cases will increase 63% from 16.52 to 27.00 million cases. Overall NAFLD prevalence among the adult population (aged ≥15 years) is projected at 33.5% in 2030, and the median age of the NAFLD population will increase from 50 to 55 years during 2015-2030. In 2015, approximately 20% of NAFLD cases were classified as NASH, increasing to 27% by 2030, a reflection of both disease progression and an aging population. Incidence of decompensated cirrhosis will increase 168% to 105,430 cases by 2030, while incidence of HCC will increase by 137% to 12,240 cases. Liver deaths will increase 178% to an estimated 78,300 deaths in 2030. During 2015-2030, there are nearly 800,000 excess liver deaths.
CONCLUSIONS:
With continued high rates of adult obesity and DM, and an aging population, NAFLD-related liver disease and mortality will increase in the US. Strategies to slow the growth of NAFLD cases and therapeutic options are necessary to mitigate disease burden.
Source: HEPATOLOGY
The negligible (mature) SREBP1a?
Posted 6/13/2017
One further newly published peer-reviewed article:
Tajima-Shirasaki et al. Eicosapentaenoic Acid Downregulates Expression
of the Selenoprotein P Gene by Inhibiting SREBP-1c Independently of the AMPK
Pathway in H4IIEC3 Hepatocytes. J Biol Chem. 2017 May 2. pii: jbc.M116.747006.
Introduction:
According to the purpose of the study, it was the aim to find out if hepatic SREBP1c induces the transcripition of SELENOP eicosapentaenoic acid (EPA)-dependently or rather if EPA, a polyunsaturated fatty acid (PUFA), inhibits the activity of SREBP1c.
Evaluation:
Without going into great detail, it is useful to consider the following three issues:
- For knock-down experiments the authors used siRNA which simultaneously targets both, the SREBP1c and SREBP1a transcript (see here). What about SREBP1a?
- In overexpression experiments the authors only transfected the precursor 1a isoform of SREBP1 but transfected separately both, the precursor AND mature isoform SREBP1c. (Only the mature SREBP1 protein is transcriptionally active). However, they have concluded: »The mature SREBP-1c overexpression especially inhibited the suppressive effect of EPA on SELENOP promoter activity (Fig. 3C); however, SREBP-1a overexpression had no effect (data not shown). These results suggest an association of SREBP-1c activity with the EPA-induced suppression of SELENOP expression.« What about the mature SREBP1a?
- In regard to the ChIP assay they have used, the authors assume: »...treatment with EPA was found to decrease the binding of SREBP-1c to Selenop promoter (Fig.5B), indicating that EPA decreases SELENOP promoter activity and gene expression via SREBP-1c inactivation...« It is important to know that SREBPs function as dimers in which the individual molecules can form homo- or heteromers (see here). What about the mature SREBP1a?
| < Previous |
